" CHEMISTRY "
Question :- What is Chemistry ?
Answer :- Chemistry is the science of matter, it's properties, structure, composition and it's changes during interactions and chemical reactions.Chemistry is sometimes called "the central science" because it connects Physics with other natural sciences such as astronomy, geology and biology.
1) ATOM :- JOHN DALTON first discovered the atomic theory i.e an atom .An atom is the basic unit of chemistry. It consists of a positively charged core (the atomic nucleus) which contains protons and neutrons, and which maintains a number of electrons to balance the positive charge in the nucleus. The atom is also the smallest entity that can be envisaged to retain some of the chemical properties of the element, such as electronegativity, ionization potential, preferred oxidation state(s), coordination number, and preferred types of bonds to form (e.g., metallic, ionic, covalent).
JOHN DALTON
An Atom
ELEMENTS :- The concept of chemical element is related to that of chemical substance. A chemical element is specifically a substance which is composed of a single type of atom. A chemical element is characterized by a particular number of protons in the nuclei of its atoms. This number is known as the atomic number of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium. Ninety–four different chemical elements or types of atoms based on the number of protons exist naturally. A further 18 have been recognised by International Union of Pure and Applied Chemistry (IUPAC) as existing artificially only. Although all the nuclei of all atoms belonging to one element will have the same number of protons, they may not necessarily have the same number of neutrons; such atoms are termed isotopes. In fact several isotopes of an element may exist.
HYDROGEN CARBON ELEMENT
COMPOUND :- A compound is a substance with a particular ratio of atoms of particular chemical elements which determines its composition, and a particular organization which determines chemical properties. For example, water is a compound containing hydrogen and oxygen in the ratio of two to one, with the oxygen atom between the two hydrogen atoms, and an angle of 104.5° between them. Compounds are formed and interconverted by chemical reactions.
COMPOUND OF WATER
BASICS OF A ATOM
Chemical element :- A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus.Familiar examples of elements include gold, iron, copper, carbon, silicon, mercury, sodium, calcium, hydrogen, nitrogen, chlorine, and neon.
Atomic number :- The atomic number of an element, Z, is equal to the number of protons that defines the element. For example, all carbon atoms contain 6 protons in their nucleus; so the atomic number "Z" of carbon is 6. Carbon atoms may have different numbers of neutrons; atoms of the same element having different numbers of neutrons are known as isotopes of the element.
Atomic mass :- The mass number of an element, A, is the number of nucleons (protons and neutrons) in the atomic nucleus. Different isotopes of a given element are distinguished by their mass numbers, which are conventionally written as a super-index on the left hand side of the atomic symbol.
Isotopes :- Isotopes are atoms of the same element (that is, with the same number of protons in their atomic nucleus), but having different numbers of neutrons. Most (66 of 94) naturally occurring elements have more than one stable isotope. Thus, for example, there are three main isotopes of carbon. All carbon atoms have 6 protons in the nucleus, but they can have either 6, 7, or 8 neutrons. Since the mass numbers of these are 12, 13 and 14 respectively, the three isotopes of carbon are known as carbon-12, carbon-13, and carbon-14, often abbreviated to 12C, 13C, and 14C. Carbon in everyday life and in chemistry is a mixture of 12C, 13C, and 14C atoms.
Allotropes :- Atoms of pure elements may bond to each other chemically in more than one way, allowing the pure element to exist in multiple structures (spacial arrangements of atoms), known as allotropes, which differ in their properties. For example, carbon can be found as diamond, which has a tetrahedral structure around each carbon atom; graphite, which has layers of carbon atoms with a hexagonal structure stacked on top of each other; graphene, which is a single layer of graphite that is incredibly strong; fullerenes, which have nearly spherical shapes; and carbon nanotubes, which are tubes with a hexagonal structure (even these may differ from each other in electrical properties). The ability for an element to exist in one of many structural forms is known as 'allotropy'.
" CLASSIFICATION OF ELEMENTS "
Johann Wolfgang Dobereiner
Döbereiner's Triads are groups of three elements having similar properties. They are named after the name of the German chemist Johann Wolfgang Döbereiner. He observed that when three elements of any particular triad were arranged in order of their increasing atomic masses, the atomic mass of the middle element was roughly the mean or average of the atomic masses of the other two elements.He was the first to classify elements into groups based on John Dalton's assertions. He grouped the elements with similar chemical properties into clusters of three called 'Triads' in 1829.
1) All the elements known at the time could not be classified as Traids ( i.e All applicable to a few elements only )
2) It does not take into account the isotopes of elements.
3) It cannot be applied to other chemically similar elements.
For Example ---
---------------------------------------------------------------
JOHN NEWLAND
John Newlands was an English chemist who in 1865 classified the 56 elements that had been discovered at the time into eleven groups which were based on similar physical properties. When the elements are arranged in increasing order of their atommic masses, the properties of every eighth element are similar to the first, as in the octave of music.
Limitations of Newlands Law of Octaves
1) Newlands law was applicable to elements with low atomic masses only. He could arrange elements only upto calcium out of total 56 elements known.
2) The law fails to explain similaraties in properties of elements with higher atomic weights. After Calcium, every eighth element did not possess properties of the first.
3) Newlands thought only 56 elements existed, but later several elements were discovered.
4) In order to fit the existing elements, Newland adjusted two elements in the same position which differed in their properties.
5) This Periodic Table did not include inert gases as they were not discovered.
In the above table , Ti is the eighth element with respect to Al, but does not show similar properties. Similarly, Cr is different from Si. The elements Ti,Cr,Mn,Fe and Co,Ni do not show similarities as per Newlands Law of Octaves.
-----------------------------------------------------------------------------
Dmitri Ivanovich Mendeleev
Dmitri Ivanovich Mendeleev was a Russian chemist and inventor. He is credited as being the creator of the first version of the periodic table of elements. Using the table, he predicted the properties of elements yet to be discovered.
Mendeleev's Periodic Law :- The physical and Chemical properties of elements are periodic function of their atomic masses.
Mendeleev's Periodic Table
Merits of Mendeleevs Periodic Table.
1) Mendeleev was the first who successfully classified all known elements .
2) Mendeleev kept some blank places in his periodic table. These vacant spaces were for elements that yet to be discovered. He also predicted properties of these elements even before they were discovered. Later they found to be correct.
3) In his periodic table, some gaps were left by Mendeleev for unknown elements that could be found in the future. Three such unknown elements were names as Eka-Boron, Eka-Aluminium, Eka-Silicon. Even the properties of these unknown elements were predicted and these were found to be accurate.
Comparision of properties of eka-aluminium and gallium.
4) When the noble gases were discovered later, They were placed in Mendeleev's Periodic table without disturbing the positions of other elements.
-----------------------------------------------------------------------------
MODERN PERIODIC TABLE
HENRY MOSELEY
HENRY MOSELEY :- Henry Gwyn Jeffreys Moseley (23 November 1887 – 10 August 1915) was an English physicist.In 1913, Henry Moseley found that it was atomic number (Z) and not atomic mass which was the fundamental property of an element. Thus, a new periodic table was made which we called today as Modern Periodic Table or long form of periodic table.
MODERN PERIODIC TABLE Law :- The physical and Chemical properties of elements are periodic function of their atomic number.
Introduction to Modern Periodic Table of Elements:
The universe is made of matter and energy. Matter can be elements, compounds and mixtures. Elements are the simplest form of matter which cannot be reduced further. Periodic table is a tool which helps us to have a over view of all the 109 existing elements.
Basics of Periodic Table of Classification of Elements
The elements in the periodic table are arranged in the ascending order of their atomic number. Atomic number is the number of electrons present in an atom of an element.
Atoms of all the elements try to get 8 electrons in their outer most shell (valence shell) of the atom (Octet rule). In doing so, each atom gain or lose /share electrons with the atoms of other elements resulting in the formation of compounds.
The atoms of rare gases or noble gases have their outermost shell (valence shell) completely filled. Hence they do not react.
Structure of Modern Periodic Table of Element
1) The periodic table of elements consists of horizontal rows called periods and vertical columns called groups.
2) There are 18 groups and 7 periods in the periodic table.
3) The elements of group 1 have one valence electron; group 2 elements have 2 valence electrons and so on.
4) Zero group elements have 8 electrons in their outermost shell.
5) Thus the elements of each group have similar properties.
6) Thus one can identify the valence electrons of the atom of the element from the group it is placed.
7) Lanthanides and actinides falling under the same group are shown separately below the table.
8) The elements in I period have their electrons placed in one shell or they have only one main shell.
9) The elements of the II period have two main shells and so on.
10) Elements on the left side of the periodic table are called metals. Elements on the right side of the periodic table are occupied by non metals. The metals and non metals are separated by metalloids. One can tell that the metals take most part of the table by glancing at it.
11) Metallic to non metallic decreases along a period of the periodic table.
Uses of Modern Periodic Table of Elements:
Knowing the atomic number, it is easy to locate and predict the chemical properties of any element in the periodic table.
The position of the element in the periodic table will help us to write its electronic configuration and identify the valence electrons in the atom of the element.
It reveals the similarities, differences and trends in chemical properties of the elements clearly.
One can easily write the molecular formula and predict the compound formed by the given two elements with the help of the periodic table.
PARTITION OF PERIODIC TABLE
Alkali Metals :- The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals are called the Group 1 elements. Previously, they were called the Group IA elements, with "A" representing the main group elements and Roman numeral "I" representing the first group within the main-group series. The alkali metals include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr).Hydrogen (H), although nominally also a member of Group 1,very rarely exhibits behaviour comparable to the alkali metals. This group lies in the s-block of the periodic table, which means that all its elements have their outermost electron in an s-orbital. The s-block also includes alkaline earth metals, plus hydrogen and helium. The alkali metals provide one of the best examples of group trends in properties in the periodic table, with well characterized homologous behaviour down the group.
LITHIUM (Li)
SODIUM (Na)
POTASSIUM (K)
RUBIDIUM (Rb)
Alkali Earth Metal :- The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements (pronounced "group two A", as the "II" here is a Roman numeral). The alkaline earth metals contain beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). (Although helium (He) is occasionally considered to be a group 2 element, for example, in the extended periodic table and Janet periodic table, it never exhibits behaviour comparable to the alkaline earth metals.) The group lies in the s-block of the periodic table.
BERRYLIUM (Be)
MAGNESIUM (Mg)
CALCIUM (Ca)
STRONTIUM (Sr)
BARIUM (Ba)
RADIUM (Ra)
Transition metal :- The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.
Characteristic properties :- 1) the formation of compounds whose colour is due to d - d electronic transitions
2) the formation of compounds in many oxidation states, due to the relatively low reactivity of unpaired d electrons.[12]
3) the formation of many paramagnetic compounds due to the presence of unpaired d electrons. A few compounds of main group elements are also paramagnetic (e.g. nitric oxide, oxygen).
The transition metals are the subgroups of elements intervening between groups IIA(2) and IIIA(13) in the periodic table. They are classified separately because of the filling of their d subshell orbitals.
All the transition elements are metallic, but unlike the representative metals, they are likely to be hard, brittle, and have high melting points because of the relatively small size of their atoms and the existence of some covalent binding between ions. There are exceptions, as in the case of mercury (Hg), which is a liquid. They have high electrical conductivity because of delocalization of the s electrons similar to what occurs in the alkali and alkaline-earth metals. Another characteristic of the transition metals is the great variety of oxidation states shown in its compounds.
Because of electron spin, unpaired electrons give rise to paramagnetism. Paramagnetism is likely in transition metals because of the partial filling of the d subshell and the movement associated with the orientations of electrons.
Most transition metals are colored and make some of their ionic compounds colored. This is because they absorb some of the frequencies of white light. This is attributed to electronic transitions in the d subshell, separating them into different levels of energy. When light is absorbed, an electron is raised from a lower state to a higher state, giving the rise to color. The stored energy is then dissipated through heat.
The transition metals also have complex ionic structures because of the availability of d orbitals for participating in chemical bonding.
INNER TRANSITION ELEMENTS :- The inner transitional metals contain 2 main groups, the lanthanide series, and the actinide series.
Question :- What is Chemistry ?
Answer :- Chemistry is the science of matter, it's properties, structure, composition and it's changes during interactions and chemical reactions.Chemistry is sometimes called "the central science" because it connects Physics with other natural sciences such as astronomy, geology and biology.
Basic of Chemistry
1) ATOM :- JOHN DALTON first discovered the atomic theory i.e an atom .An atom is the basic unit of chemistry. It consists of a positively charged core (the atomic nucleus) which contains protons and neutrons, and which maintains a number of electrons to balance the positive charge in the nucleus. The atom is also the smallest entity that can be envisaged to retain some of the chemical properties of the element, such as electronegativity, ionization potential, preferred oxidation state(s), coordination number, and preferred types of bonds to form (e.g., metallic, ionic, covalent).
JOHN DALTON
An Atom
ELEMENTS :- The concept of chemical element is related to that of chemical substance. A chemical element is specifically a substance which is composed of a single type of atom. A chemical element is characterized by a particular number of protons in the nuclei of its atoms. This number is known as the atomic number of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium. Ninety–four different chemical elements or types of atoms based on the number of protons exist naturally. A further 18 have been recognised by International Union of Pure and Applied Chemistry (IUPAC) as existing artificially only. Although all the nuclei of all atoms belonging to one element will have the same number of protons, they may not necessarily have the same number of neutrons; such atoms are termed isotopes. In fact several isotopes of an element may exist.
HYDROGEN CARBON ELEMENT
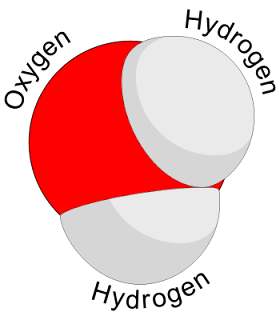
COMPOUND :- A compound is a substance with a particular ratio of atoms of particular chemical elements which determines its composition, and a particular organization which determines chemical properties. For example, water is a compound containing hydrogen and oxygen in the ratio of two to one, with the oxygen atom between the two hydrogen atoms, and an angle of 104.5° between them. Compounds are formed and interconverted by chemical reactions.
COMPOUND OF WATER
BASICS OF A ATOM
Chemical element :- A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus.Familiar examples of elements include gold, iron, copper, carbon, silicon, mercury, sodium, calcium, hydrogen, nitrogen, chlorine, and neon.
Atomic number :- The atomic number of an element, Z, is equal to the number of protons that defines the element. For example, all carbon atoms contain 6 protons in their nucleus; so the atomic number "Z" of carbon is 6. Carbon atoms may have different numbers of neutrons; atoms of the same element having different numbers of neutrons are known as isotopes of the element.
Atomic mass :- The mass number of an element, A, is the number of nucleons (protons and neutrons) in the atomic nucleus. Different isotopes of a given element are distinguished by their mass numbers, which are conventionally written as a super-index on the left hand side of the atomic symbol.
Isotopes :- Isotopes are atoms of the same element (that is, with the same number of protons in their atomic nucleus), but having different numbers of neutrons. Most (66 of 94) naturally occurring elements have more than one stable isotope. Thus, for example, there are three main isotopes of carbon. All carbon atoms have 6 protons in the nucleus, but they can have either 6, 7, or 8 neutrons. Since the mass numbers of these are 12, 13 and 14 respectively, the three isotopes of carbon are known as carbon-12, carbon-13, and carbon-14, often abbreviated to 12C, 13C, and 14C. Carbon in everyday life and in chemistry is a mixture of 12C, 13C, and 14C atoms.
Allotropes :- Atoms of pure elements may bond to each other chemically in more than one way, allowing the pure element to exist in multiple structures (spacial arrangements of atoms), known as allotropes, which differ in their properties. For example, carbon can be found as diamond, which has a tetrahedral structure around each carbon atom; graphite, which has layers of carbon atoms with a hexagonal structure stacked on top of each other; graphene, which is a single layer of graphite that is incredibly strong; fullerenes, which have nearly spherical shapes; and carbon nanotubes, which are tubes with a hexagonal structure (even these may differ from each other in electrical properties). The ability for an element to exist in one of many structural forms is known as 'allotropy'.
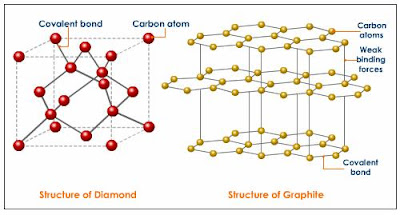 |
| Allotropes of Carbon |
---------------------------------------------------------------------------------
" CLASSIFICATION OF ELEMENTS "
Johann Wolfgang Dobereiner
Döbereiner's Triads are groups of three elements having similar properties. They are named after the name of the German chemist Johann Wolfgang Döbereiner. He observed that when three elements of any particular triad were arranged in order of their increasing atomic masses, the atomic mass of the middle element was roughly the mean or average of the atomic masses of the other two elements.He was the first to classify elements into groups based on John Dalton's assertions. He grouped the elements with similar chemical properties into clusters of three called 'Triads' in 1829.
Limitation of Dobereiner's Traids
1) All the elements known at the time could not be classified as Traids ( i.e All applicable to a few elements only )
2) It does not take into account the isotopes of elements.
3) It cannot be applied to other chemically similar elements.
For Example ---
---------------------------------------------------------------
JOHN NEWLAND
John Newlands was an English chemist who in 1865 classified the 56 elements that had been discovered at the time into eleven groups which were based on similar physical properties. When the elements are arranged in increasing order of their atommic masses, the properties of every eighth element are similar to the first, as in the octave of music.
Limitations of Newlands Law of Octaves
1) Newlands law was applicable to elements with low atomic masses only. He could arrange elements only upto calcium out of total 56 elements known.
2) The law fails to explain similaraties in properties of elements with higher atomic weights. After Calcium, every eighth element did not possess properties of the first.
3) Newlands thought only 56 elements existed, but later several elements were discovered.
4) In order to fit the existing elements, Newland adjusted two elements in the same position which differed in their properties.
5) This Periodic Table did not include inert gases as they were not discovered.
In the above table , Ti is the eighth element with respect to Al, but does not show similar properties. Similarly, Cr is different from Si. The elements Ti,Cr,Mn,Fe and Co,Ni do not show similarities as per Newlands Law of Octaves.
-----------------------------------------------------------------------------
Dmitri Ivanovich Mendeleev
Dmitri Ivanovich Mendeleev was a Russian chemist and inventor. He is credited as being the creator of the first version of the periodic table of elements. Using the table, he predicted the properties of elements yet to be discovered.
Mendeleev's Periodic Law :- The physical and Chemical properties of elements are periodic function of their atomic masses.
Mendeleev's Periodic Table
Merits of Mendeleevs Periodic Table.
1) Mendeleev was the first who successfully classified all known elements .
2) Mendeleev kept some blank places in his periodic table. These vacant spaces were for elements that yet to be discovered. He also predicted properties of these elements even before they were discovered. Later they found to be correct.
3) In his periodic table, some gaps were left by Mendeleev for unknown elements that could be found in the future. Three such unknown elements were names as Eka-Boron, Eka-Aluminium, Eka-Silicon. Even the properties of these unknown elements were predicted and these were found to be accurate.
Comparision of properties of eka-aluminium and gallium.
4) When the noble gases were discovered later, They were placed in Mendeleev's Periodic table without disturbing the positions of other elements.
-----------------------------------------------------------------------------
MODERN PERIODIC TABLE
HENRY MOSELEY
HENRY MOSELEY :- Henry Gwyn Jeffreys Moseley (23 November 1887 – 10 August 1915) was an English physicist.In 1913, Henry Moseley found that it was atomic number (Z) and not atomic mass which was the fundamental property of an element. Thus, a new periodic table was made which we called today as Modern Periodic Table or long form of periodic table.
MODERN PERIODIC TABLE Law :- The physical and Chemical properties of elements are periodic function of their atomic number.
Introduction to Modern Periodic Table of Elements:
The universe is made of matter and energy. Matter can be elements, compounds and mixtures. Elements are the simplest form of matter which cannot be reduced further. Periodic table is a tool which helps us to have a over view of all the 109 existing elements.
Basics of Periodic Table of Classification of Elements
The elements in the periodic table are arranged in the ascending order of their atomic number. Atomic number is the number of electrons present in an atom of an element.
Atoms of all the elements try to get 8 electrons in their outer most shell (valence shell) of the atom (Octet rule). In doing so, each atom gain or lose /share electrons with the atoms of other elements resulting in the formation of compounds.
The atoms of rare gases or noble gases have their outermost shell (valence shell) completely filled. Hence they do not react.
Structure of Modern Periodic Table of Element
1) The periodic table of elements consists of horizontal rows called periods and vertical columns called groups.
2) There are 18 groups and 7 periods in the periodic table.
3) The elements of group 1 have one valence electron; group 2 elements have 2 valence electrons and so on.
4) Zero group elements have 8 electrons in their outermost shell.
5) Thus the elements of each group have similar properties.
6) Thus one can identify the valence electrons of the atom of the element from the group it is placed.
7) Lanthanides and actinides falling under the same group are shown separately below the table.
8) The elements in I period have their electrons placed in one shell or they have only one main shell.
9) The elements of the II period have two main shells and so on.
10) Elements on the left side of the periodic table are called metals. Elements on the right side of the periodic table are occupied by non metals. The metals and non metals are separated by metalloids. One can tell that the metals take most part of the table by glancing at it.
11) Metallic to non metallic decreases along a period of the periodic table.
Uses of Modern Periodic Table of Elements:
Knowing the atomic number, it is easy to locate and predict the chemical properties of any element in the periodic table.
The position of the element in the periodic table will help us to write its electronic configuration and identify the valence electrons in the atom of the element.
It reveals the similarities, differences and trends in chemical properties of the elements clearly.
One can easily write the molecular formula and predict the compound formed by the given two elements with the help of the periodic table.
PARTITION OF PERIODIC TABLE
Alkali Metals :- The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals are called the Group 1 elements. Previously, they were called the Group IA elements, with "A" representing the main group elements and Roman numeral "I" representing the first group within the main-group series. The alkali metals include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr).Hydrogen (H), although nominally also a member of Group 1,very rarely exhibits behaviour comparable to the alkali metals. This group lies in the s-block of the periodic table, which means that all its elements have their outermost electron in an s-orbital. The s-block also includes alkaline earth metals, plus hydrogen and helium. The alkali metals provide one of the best examples of group trends in properties in the periodic table, with well characterized homologous behaviour down the group.
LITHIUM (Li)
SODIUM (Na)
 |
POTASSIUM (K)
RUBIDIUM (Rb)
Alkali Earth Metal :- The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements (pronounced "group two A", as the "II" here is a Roman numeral). The alkaline earth metals contain beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). (Although helium (He) is occasionally considered to be a group 2 element, for example, in the extended periodic table and Janet periodic table, it never exhibits behaviour comparable to the alkaline earth metals.) The group lies in the s-block of the periodic table.
BERRYLIUM (Be)
MAGNESIUM (Mg)
CALCIUM (Ca)
STRONTIUM (Sr)
BARIUM (Ba)
RADIUM (Ra)
Transition metal :- The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.
Characteristic properties :- 1) the formation of compounds whose colour is due to d - d electronic transitions
2) the formation of compounds in many oxidation states, due to the relatively low reactivity of unpaired d electrons.[12]
3) the formation of many paramagnetic compounds due to the presence of unpaired d electrons. A few compounds of main group elements are also paramagnetic (e.g. nitric oxide, oxygen).
The transition metals are the subgroups of elements intervening between groups IIA(2) and IIIA(13) in the periodic table. They are classified separately because of the filling of their d subshell orbitals.
All the transition elements are metallic, but unlike the representative metals, they are likely to be hard, brittle, and have high melting points because of the relatively small size of their atoms and the existence of some covalent binding between ions. There are exceptions, as in the case of mercury (Hg), which is a liquid. They have high electrical conductivity because of delocalization of the s electrons similar to what occurs in the alkali and alkaline-earth metals. Another characteristic of the transition metals is the great variety of oxidation states shown in its compounds.
Because of electron spin, unpaired electrons give rise to paramagnetism. Paramagnetism is likely in transition metals because of the partial filling of the d subshell and the movement associated with the orientations of electrons.
Most transition metals are colored and make some of their ionic compounds colored. This is because they absorb some of the frequencies of white light. This is attributed to electronic transitions in the d subshell, separating them into different levels of energy. When light is absorbed, an electron is raised from a lower state to a higher state, giving the rise to color. The stored energy is then dissipated through heat.
The transition metals also have complex ionic structures because of the availability of d orbitals for participating in chemical bonding.
INNER TRANSITION ELEMENTS :- The inner transitional metals contain 2 main groups, the lanthanide series, and the actinide series.
The Lanthanides :- The lanthanide series include the 14 elements that proceed lanthanum (atomic number 57) from atomic numbers 58 to 71. Their electron configuration include the 4f and 5d energy levels. Because of the closeness of those two levels, there is considerable uncertainty in some electron configuration assignments. All the lanthanides form +3 ions as their principal chemical species. It is assumed that the ions are formed by losing the 6s2 and 5d1 (or 4f is 5d is not present) electrons. They generally occur together, except for promethium, which has an unstable nucleus. The richest source mineral is monazite, a complex phosphate. They are very rare to find, hence their nickname--"the rare earth elements." Since they also have very similar chemical properties, separation is very difficult, involving fractional crystallization and ion-exchange techniques. The lanthanides also generally have an incomplete 4f subshell, resulting in paramagnetism.
The Actinides :- The actinide series include the 14 elements that proceed actinium (atomic number 89) from atomic numbers 90 to 103. The electron configurations of the actinides are even more uncertain than the lanthanides because the closeness of the energy levels and because the nuclei are unstable to radioactive decay. Only minute amounts of some elements are obtained because of their instability. All of the actinides are unstable with respect to alpha emission. The later members tend to undergo spontaneous fission, a fact which limits the number of elements possible. The actinides also seem to show a variety of oxidation states, unlike the lanthanides. Uranium, for example, has compounds in each of the states, +3, +4, +5, and +6
Halogens :- The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style (formerly: VII, VIIA) of the periodic table, comprising fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). The artificially createdelement 117, provisionally referred to by the systematic name ununseptium, may also be a halogen.The group of halogens is the only periodic table group which contains elements in all three familiar states of matter at standard temperature and pressure.
Inert Gases :- An inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration. Neon and argon are the most common inert gases for use in chemistry and archival settings.Unlike noble gases, an inert gas is not necessarily elemental and is often a compound gas. Like the noble gases the tendency for non-reactivity is due to the valence, the outermost electron shell, being complete in all the inert gases.[1] This is a tendency, not a rule, as noble gases and other "inert" gases can react to form compounds.
In marine applications, the term "inert gas" is used for gases with a low content of oxygen that are used to fill void spaces in and around tanks for explosion protection. There are two types of inert gas which are either based on nitrogen or on flue gas.
In underwater diving an inert gas is a component of the breathing mixture which is not metabolicaly active, and serves to dilute the gas mixture. The inert gas may have effects on the diver, but these are thought to be mostly physical effects, such as tissue damage caused by bubbles in decompression sickness. The most common inert gases used in breathing gases for diving are nitrogen and helium.
In marine applications, the term "inert gas" is used for gases with a low content of oxygen that are used to fill void spaces in and around tanks for explosion protection. There are two types of inert gas which are either based on nitrogen or on flue gas.
In underwater diving an inert gas is a component of the breathing mixture which is not metabolicaly active, and serves to dilute the gas mixture. The inert gas may have effects on the diver, but these are thought to be mostly physical effects, such as tissue damage caused by bubbles in decompression sickness. The most common inert gases used in breathing gases for diving are nitrogen and helium.
DISCHARGE TUBES OF INERT GASES
 |
| KRYTON |
 |
| NEON |
 |
| ARGON |
 |
| HELIUM |
-------------------------------------------------------------------------------------------------